Bovine Viral Diarrhoea (BVD)
Bovine viral diarrhoea (BVD) is a common infectious disease among cattle worldwide and the BVD virus has health and welfare implications as well as causing economic damage on cattle farms. Efforts to tackle BVD started in 1998 with a voluntary ‘BVD-free’ programme. The approach to BVD progressed to a new nationwide phase from 1 April 2018.
Animal disease information for Bovine Viral Diarrhoea (BVD)
The virus
The virus that causes bovine viral diarrhoea belongs to the Pestivirus genus of the family Flaviviridae. Both Border Disease Virus (BDV) and Classical Swine Fever virus (CSF) are also members of the Pestivirus genus. Based on the genetic differences, the Pestivirus genus has been classified into four separate groups: BVDV, CSFV, BDV and an atypical group.
The BVD virus (BVDV) is split into two types, based on genetic differences and antigenic characteristics, namely BVDV-1 and BVDV-2. Both types are then further subdivided into genetic subtypes such as BVDV-1b (see Figure 2, Peterhans et al. 2010). The most commonly occurring variant in the Netherlands is BVDV type 1. The BVD virus occurs primarily in cattle but infections also occur in other animal species, including pigs and small ruminants.
Irrespective of the BVDV type (1 or 2), there are 2 different biotypes of BVDV. Based on the cytopathogenic effect on cell cultures, BVDV is classified as either cytopathogenic (cp) or non-cytopathogenic (ncp). The cytopathogenic biotype (cp) caused vacuolization of the cytoplasm and cell degeneration, in contrast to the non-cytopathogenic biotype (ncp). The ncp viruses are responsible for transplacental infections that result in abortion, congenital abnormalities and births of persistently infected animals (PI). Genetic recombination can lead to cp viruses arising from ncp viruses. The combination of cp and ncp viruses in a PI animal leads to mucosal disease (MD).
Transient infection (TI)
Ingestion of BVDV (ncp biotype) is generally through the oronasal pathway. After ingestion, BVDV multiplies in the tonsils after which it spreads and invades lymphocytes and macrophages in the local lymph nodes. BVDV has direct negative effects on immune cells, which explains why transiently infected animals exhibit short-term leukopenia, lymphopenia and sometimes thrombocytopenia in the acute phase. Viraemia may subsequently occur two to four days after infection; various organs and tissues may be infected thereafter, depending on how virulent the BVDV type is. BVDV is then detectable in the blood and all the animal’s secretions and excretions contain the virus.
The symptoms when an immunocompetent animal is infected can vary, depending on the virulence of the BVDV type, from mild through to severe with high mortality. In transient infections, there is always immunosuppression due to the combination of the innate immune response being compromised plus lymphopenia. This leads to the animals being more susceptible to secondary infections.
The period during which transiently infected animals secrete the virus depends on the virulence of the BVDV type and the efficiency of replication, but generally varies from seven to at most seventeen days. Even after the systemic immune response and elimination of BVDV from the blood, it is possible that transiently infected animals may still temporarily excrete the virus as a consequence of sequestration of the virus. BVDV can be detected on nasal swabs for up to 31 days after infection and in exceptional cases it may remain detectable for a long time in the serum (80 days) and ear biopsies (more than 80 days) and in the semen of TI bulls (over two years).
The humoral immune response in TI animals is characterized by the presence of antibodies from two to three weeks after infection. The animals remain seropositive for life after infection, i.e. meaning for the rest of their lives in the case of animals kept for commercial purposes.
Infection during gestation
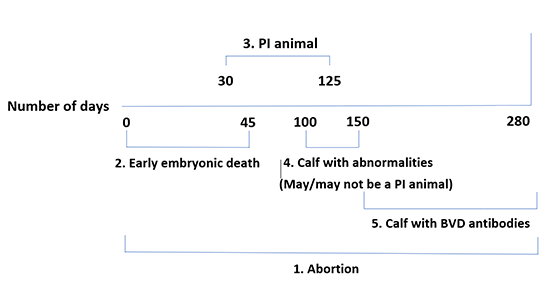
- Death of the foetus followed by mummification, resorption or abortion; this can occur at any point during the gestation.
- Early death of the embryo up to 45 days after conception; this is often not noticed.
- Birth of a persistently infected calf (PI calf). This happens if the foetus becomes infected between day 30 and day 125 of gestation. Because infection in such cases occurs while the calf’s immune system is not yet developed, the BVD virus is treated as endogenous and the calf will therefore not produce antibodies.
- Birth of a calf with visually detectable abnormalities, for instance in the eye, hair coat or brain. These animals have usually become infected between Day 100 and Day 150 of gestation. The conditions described include hypomyelinogenesis, hydrocephalus, cerebellar hypoplasia, retinal atrophy and microphthalmia. These abnormalities occur in both carriers and non-carriers.
- Birth of a calf with precolostral antibodies. In the case of infection during the final three months of gestation, the immune system is sufficiently developed that the calf can eliminate a BVD infection and produce antibodies. However, it is still possible for abortion to occur or for abnormal or small, weak calves to be born.
Persistent infection (PI)
The uterus, placenta and foetus are all easily infected during an infection with BVDV. Depending on the BVDV biotype and the gestational age, a BVDV infection of the foetus can result in early death of the embryo, abortion, congenital malformations, the birth of a PI calf or the birth of a healthy calf. If infection of the foetus happens between days 30 and 125 of gestation, the foetus recognizes viral proteins as endogenous antigens, given that the foetus is not yet immunocompetent at that stage. This results in the birth of a persistently infected calf. These PI calves may apparently be healthy at birth but will continue to secrete BVDV for life. PI calves are tolerant of their ‘own’ ncp BVDV type but are however immunocompetent against other pathogens including other BVDVs. The immune response is often less effective and the life expectancy of PI animals is mostly low. In the Netherlands, more than 90 per cent of PI calves die before reaching two years of age.
Pregnant PI cows pass BVDV on to their offspring, resulting in a new generation of PI calves being born. It is therefore recommended that the mothers of PI animal should also be tested for the BVD virus. Semen from PI bulls contains large quantities of BVDV; fertilization with this sperm can cause an acute infection in a seronegative cow and may ultimately result in the birth of a PI calf.
Mucosal disease can occur in PI animals if they become infected with cp BVDV. Mucosal disease is a severe form of BVD with a high mortality rate. The cp biotype can arise by mutation of the ncp biotype that the PI animal is a carrier of. It is also possible for a PI animal to be infected by a homologous cp BVDV, for instance from another PI animal that is affected by mucosal disease.
Epidemiology
The transmission rate of infectious agents within a herd depends on the reproduction ratio (R), which is defined as the number of animals that can be infected by a single contagious animal within a susceptible population. If the value of R is less than unity, the infection will die out given that less than one other animal is infected on average by a contagious animal. A condition can therefore spread and perhaps result in an outbreak – even a major one – if R > 1 but will die out if R < 1. The reproduction ratio is determined by the following parameters: β = the probability of transmission, к = the frequency of contact with naive individuals and D = the duration of the infectious interval (where R = βкD). PI animals secrete large quantities of BVDV continuously throughout their lives, resulting in high values for the parameters β and D. TI animals secrete much less of the virus and also only do so for a limited interval, resulting in low values for the parameters β and D. This means that PI animals play a crucial role in the epidemiology of BVD.
When a PI animal is introduced into a susceptible herd, BVDV will spread within a few weeks or months until about 90 per cent of the animals have had an infection and produced antibodies as a result. The speed at which the virus spreads within a farm depends strongly on the parameter к.
If BVDV is introduced at a farm when a PI animal is introduced into a susceptible herd, a primary infection will develop in the first instance. Depending on the virulence of the BVDV, this will be associated with a variable number of clinical symptoms. If a substantial proportion of the herd is pregnant at the time that BVDV is introduced, this will result in a (limited) number of abortions a few weeks later, possibly followed five to eight months later by one or more PI animals being born. If these PI animals are not removed from the herd quickly, the spread of the virus on the farm will be maintained and problems may persist for a long time (up to two years) as a result.
Symptoms
The clinical symptoms of BVD depend on:
- the immunity and fertility status of the infected animal
- BVDV virulence factors
- the presence of secondary/concomitant infections
The consequence is nevertheless that the clinical symptoms can range from moderate to severe with high mortality rates, making it difficult to recognize BVD based on the clinical symptoms alone.
Acute infection
It is estimated that around 70 to 90 per cent of acute BVD infections in immunocompetent, seronegative animals are associated with limited clinical symptoms. Transient infections in adult animals are therefore by no means always noticed. A proportion of acute BVD infections are however associated with clinical symptoms to a greater or lesser extent. The following clinical symptoms may be observed:
- a drop in production (up to 10 per cent in subclinically infected cows)
- Inflammation of the mucous membranes, causing mucosal erosion and mucosal ulceration
- BVDV can get into enterocytes, which can cause diarrhoea
- fever
- loss of appetite and/or dehydration
- abortion and fertility disorders
- death
In rare cases, transient BVD infections can cause acute or peracute outbreaks with clinical signs such as fever, pneumonia and haemorrhagic syndrome (HD). Cases of haemorrhagic syndrome are typified by severe thrombocytopenia and coupled with an elevated tendency to bleed, resulting in black or bloody diarrhoea and petechiae (on the oral mucosa and the tongue). Animals with this clinical picture mostly die within 48 hours. The majority of HD outbreaks are caused by BVDV-2. Acute outbreaks coupled with severe clinical symptoms have however also been described for infections with BVDV-1.
Immunosuppression
Despite the fact that the course of BVD infections is often subclinical, immunosuppression occurs in all transiently infected animals because BVDV infects cells of the immune system and will have a negative effect on those cells. This means that the animals are more easily infected by secondary pathogens. Co-infections with secondary pathogens can cause economic damage as a result of e.g. bovine respiratory disease or mastitis. More mastitis can be seen in herds of cows that have been in contact with BVDV and the bulk somatic cell count can be higher than in naive herds. BVDV plays a major role in BRD syndrome given that synergistic effects have been demonstrated for co-infections of BVDV with BRSV, IBR, PI-3, Mycoplasma bovis and Mannheimia haemolytica.
Calves
Due to the immunosuppressing characteristics of BVDV, secondary infections can easily affect transiently infected calves. A BVD infection in a calf can cause secondary diarrhoea and respiratory disease problems. The mortality rate among calves (< 1 year) is higher in BVDV-seropositive herds compared to seronegative herds.
Persistently infected animals
In addition to a reduced immune response and shorter life expectancy, persistently infected calves may also have clinical abnormalities at birth. Abnormalities that are commonly seen include neurological problems as a result of brain abnormalities (shaking or seizures, opithotonus), eye abnormalities (including blindness), dermatological abnormalities (hair too long and curly) and growth retardation.
Mucosal disease
In the acute form of MD, animals die within seven to fourteen days. This is preceded by a severe clinical picture comprising fever, anorexia, lameness, hoof lesions, profuse and watery or sometimes bloody diarrhoea, and lesions of the oral cavity mucosa, oesophagus and gastrointestinal tract. The lesions may extend into the submucosa. There is no treatment. In peracute cases, the animals may die before the clinical symptoms are observable. In exceptional cases, a PI animal can be infected with a cp virus with an antigen structure that differs from the PI animal’s virus. This causes a chronic form of MD with intermittent diarrhoea; the animals waste away over a period of a few weeks to eighteen months.
Diagnosing BVD
Diagnoses of BVD based on clinical symptoms are unreliable and need to be confirmed by diagnostic testing. BVD infections can be demonstrated in various ways:
- Directly, by detecting BVDV or viral components. GD has the following tests available for the purpose:
- BVD antigen ELISA, on serum (from 31 days old), ear biopsies or organs
- BVD virus PCR, on bulk milk and serum
- BVD virus isolation, on serum and semen
- BVD genotyping PCR, on serum, bulk milk and tissues
- Indirectly, by detecting the immune response through the presence of antibodies. GD has the following tests available for the purpose:
- BVD antibody ELISA, on serum and bulk milk
- BVD antibody ELISA titration, on serum
Detection of BVD and viral components
Antigen Enzyme-Linked ImmunoSorbent Assay (Ag-ELISA)
Ag-ELISA allows BVD antigen to be detected in serum, organs or ear biopsies. The Ag-ELISA used by GD targets the envelope Erns glycoprotein. The test detects both BVDV types 1 and 2. Because this protein is secreted in relatively large amounts by infected cells and is also relatively well conserved during that process, it can be detected well in serum and in ear biopsies. The sensitivity of the test is 99-100 per cent. The specificity is 99.5 per cent. The Ag-ELISA technique is reliable and relatively affordable; false negative results can however occur in serum due to the presence of maternal antibodies. Maternal antibodies can eliminate the antigen, meaning that it is then not available for the test. Serum from animals aged under 31 days is therefore unsuitable for investigation using Ag-ELISA.
It is also possible to use Ag-ELISA for ear biopsies. Given that there is no contact with the blood when taking the ear biopsy, the presence of maternal antibodies does not affect the outcome of this test. Ear biopsies can therefore be taken and examined at a very young age (directly after birth). Special ear tags are needed for taking an ear biopsy; ear tag manufacturers have these biopsy ear tags in their product ranges.
Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
The PCR test is used for detecting the RNA of BVDV (types 1 and 2) in bulk milk or serum. The high sensitivity (100 per cent) of this test allows RNA from BVDV to be detected in material with small quantities of BVDV RNA. RT-PCR is therefore suitable for examining bulk milk samples (from a maximum of 300 milk cows). If bulk milk from more than 300 milk-producing animals has to be investigated, a non-standard protocol is used. RT-PCR is insensitive to maternal antibodies and the test is therefore also suitable for examinations (including blood tests) of calves younger than one month (where the samples are not pooled). For older animals, serum can be tested in pools of 15 samples. The specificity of the serum test is 99 to 100 per cent. The relative specificity for detecting PI animals in bulk milk is 96 to 100 per cent. This is caused by the fact that the bulk milk may occasionally give a positive PCR response caused by the occurrence of acute infections in the herd before any PI animals have been born.
Virus isolation
Virus isolation allows live BVD virus particles to be detected by inoculating cell cultures with sample material and subjecting them three to five days later to immunofluorescence or immunoperoxidase staining. This allows virus isolation to be used for detecting BVDV in blood (or blood serum) and semen. This test is sometimes used in post-mortem material to diagnose mucosal disease (in which cytopathogenic BVDV is detected).
PCR for BVD genotyping
Real-time PCR can be used to distinguish between BVD genotypes 1 and 2. PCR can be used in follow-up diagnostic testing for genotyping individual BVD-positive samples (of serum, bulk milk or tissue).
Detecting BVD antibodies
Antibody ELISA (Ab-ELISA)
Ab-ELISA is used for detecting BVD in serum or bulk milk. It is also possible to carry out both paired and unpaired blood tests using ELISA titration. A titre of ≥2 means that BVD antibodies have been detected. ELISA screening shows whether antibodies are present or not. The sensitivity and specificity of individual serum tests are 98 and 99.2 per cent respectively. The tipping point in bulk milk testing from ‘detected in bulk milk’ to ‘not detected’ is at about 30 per cent of the milk cows being infected.
Relationship between vaccination and BVD diagnostics
The Ab-ELISA used at GD is an NS3-blocking ELISA targeting the NS3 protein (also known as p80). NS3 is a non-structural protein that is not part of the virus envelope; it is responsible for splitting the viral polyprotein into separate parts and it is needed for infectious virus particles to be formed. The NS3 protein is therefore essential for virus replication and is only expressed during replication of BVDV inside the host cell. This means that antibodies to NS3 are detected after an infection in the field and after vaccination with a live vaccine. After vaccination with an inactive vaccine, antibodies are scarcely detectable (if at all) because the inactivated viral particles from the vaccines do not get replicated. The more often an animal has been vaccinated with an inactivated vaccine, the greater the likelihood of a short-term antibody reaction in the ELISA test.
When a live vaccine is used, this should be taken into account in the virus diagnostics. After vaccination with a live vaccine, BVDV can be detected in the blood and milk for up to 23 days, and for a similar period in ear biopsies of calves born from vaccinated mothers.
Factors to consider in BVD testing
The following test methods are available for detecting the BVD virus in young calves:
- Newborn calf: ear biopsy test using Ag-ELISA. The ear biopsy is taken when the ear tag is applied.
- Blood test in animals young than 31 days: individual PCR. Because of the possibility of interference from maternal antibodies that can affect the outcome, Ag-ELISA is not suitable for animals younger than 1 month.
- Blood test in animals older than 31 days: PCR blood test or antigen ELISA. For cost reasons, the Ag-ELISA test is usually chosen.
For detecting a BVD infection in older calves and adult animals, the following points are important:
- A transient infection in juvenile and mature cattle can be detected by carrying out paired serological testing using ELISA titration with an interval of at least three weeks.
- Potential PI animals can be detected using Ag-ELISA. To determine whether an animal’s infection is persistent or transient, retesting for BVDV is needed (after an interval of at least three weeks). There are two possibilities, depending on the result of the retest:
- Retesting shows the virus once again: the animal in question is a PI animal.
- Retesting does not show the virus: the animal in question has had a BVD infection. The animal has therefore been in contact with the BVD virus but has overcome the BVD infection and produced antibodies. It should be noted here that it may be longer than three weeks before antibodies can be detected.
Abortion diagnostics
In the event of an abortion, a diagnosis can be made by virological and serological testing of the foetus and/or the mother animal.
The result of serological blood testing of the mother animal is assessed as follows:
- No antibodies detected: this excludes BVD as the cause of abortion. It is possible for a mother animal with no BVD antibodies in the blood to be a BVD virus carrier but this is however the exception rather than the rule in cases of abortion.
- Antibodies shown to be present: over the course of time, the number of animals with antibodies has dropped further and further (if unvaccinated). Detecting antibodies means at any rate that the animal has been exposed to BVDV. There is little point in paired serological testing of the mother animal, given that the infection will in most cases have happened some time ago and the animals already have antibodies by the time abortion occurs.
The virological tests of the aborted foetus are assessed as follows:
- If BVDV is detected by Ag-ELISA, PCR testing is done for confirmation. If BVDV is shown to be present in both tests, it indicates an infection in utero.
- If BVDV is not detected, it can mean that:
- BVD did not play a role.
- The foetus overcame the infection and eliminated the virus.
- That BVDV could not be shown to be present because of the condition of the foetus.
Prevalence
Netherlands - BVD programmes
Programmes to control BVD have been in place since 1998. These were initially voluntary but cattle farms that supply milk to any Dutch dairy plant that is affiliated to the Nederlandse Zuivel Organisatie (NZO, Dutch Dairy Organization) have been obliged since April 2018 to take part in a BVD programme to make them free of BVD. In 2012, the percentage of farms that were free of BVD or where it was not suspected was around 30 per cent. In the years that followed, the percentage of farms that were free of BVD or where it was not suspected rose to 47 per cent in August 2016 (2013: 31 per cent, 2014: 36 per cent, 2015: 38 per cent). By the second quarter of 2023, 98.8% of all dairy farms were taking part in the BVD control measures and 90% of those farms had a favourable status (BVD-free or BVD not suspected). 23% of non-dairy farms took part in the control programme. Of those farms, 85.1% had a favourable status. In 2019, the estimated nationwide prevalence for suckler cows was 7.5% [95% confidence interval: 4.3-11.9] and for small cattle holdings it was 12.0% [95% confidence interval: 6.1-20.4].
Abroad
Because BVD infections can cause major economic losses, various European countries have started mandatory control and certification programmes.
The Scandinavian countries have already been tackling the eradication of BVDV for some time. Finland, Norway, Sweden and Denmark started BVD control programmes in 1993-1994 and those countries are now free of BVD. The programmes in those countries did not involve vaccination and it proved possible to eradicate BVD without using vaccines. Austria started a voluntary programme in 1997 and adopted a nationwide approach in 2004 similar to that used in Sweden.
Since the introduction of BVDV testing of ear biopsies, other countries have also started BVD control programmes, such as the one in Switzerland for instance. A nationwide control programme commenced in Germany in 2011, adopting a scenario of eliminating PI animals combined with vaccination. Vaccination is recommended in France in addition to detecting PI animals. A mandatory nationwide BVD control programme was launched in Belgium in 2015.
Under the European Union’s Animal Health Law, BVD is a category C+D+E disease. That means that EU countries decide for themselves whether they want to eradicate the disease (C), but are required to contain it via international traders or transporters or travellers (D), and the disease must be monitored and reported on quarterly (E).
EU member states and EU zones with a disease-free status (as per the most recent update on 27 October 2021) are Austria, Denmark, Finland, Sweden and parts of Germany. EU member states and EU zones with an approved eradication programme are Ireland and parts of Germany.
Approach to tackling BVD
Netherlands - control programme
All companies that supply milk and are affiliated to the NZO are required to take part in the programme for becoming BVD-free. The programme is run by ZuivelNL and conducted by GD. There are four paths that can be used:
- BVD Route intake virus surveillance of young stock antibodies;
- BVD Route bulk milk;
- BVD Route young stock antibodies;
- BVD Route ear notch testing.
In brief:
- BVD Route: intake virus, surveillance of young stock antibodies – monitoring antibodies in young stock: all animals are given a one-off test for the virus (animals supplying milk can be tested via bulk milk tests for the virus) and all new calves born over a ten-month period are tested for the virus using ear biopsies. All PI animals found are removed from the herd. The ten-month period recommences after a PI animal is removed from the herd. After the intake phase is completed, the farm gets the status “BVD-free” and six-monthly monitoring commences using random sampling of young cattle aged from 8 to 12 months.
- BVD Route: bulk milk – bulk milk testing of farms without BVD antibodies, 4 times a year. The status “BVD not suspected” is given after one favourable bulk milk test result. After a minimum of 24 months with favourable bulk milk test results, the farm gets the status “BVD-free”.
- BVD Route: surveillance of young stock antibodies – farms do six-monthly sampling among young stock, testing five animals aged between 8 and 12 months for BVD antibodies. The farm is given the status “BVD not suspected” after a single favourable random test. After a minimum of 24 months with favourable random test result from young animals, the farm gets the status “BVD-free”.
- BVD Route: ear notch testing – for ten months, all newborn calves are tested for BVDV using ear biopsies. All PI animals found are removed from the herd.. The ten-month period recommences after a PI animal is removed from the herd. The farm is given the status “BVD not suspected” after ten months. Taking ear biopsies continues for monitoring purposes. After favourable virus results for a minimum of 34 consecutive months, the farm gets the status “BVD-free”.
- An essential part of the monitoring is examining animals on arrival (after purchase) from farms that do not have the BVD-free status.
For the currently applicable protocols, please refer to the page "Bestrijding IBR & BVD: protocollen" from ZuivelNL (in Dutch).
Non-dairy farms can also take part in the programme on a voluntary basis.
General principles
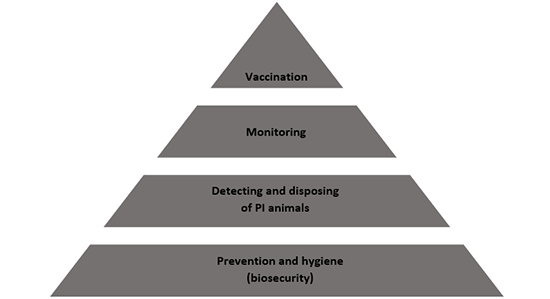
For tackling BVD on farms effectively and successfully, it is important to focus on certain specific measures. A general model for combating BVD successfully is then based on three cornerstones:
- Biosecurity and prevention
- Detecting and disposing of PI animals
- Monitoring
Vaccination can be added as a fourth element as long as the first three elements are complied with. The four cornerstones are shown schematically below.
Biosecurity and prevention
Biosecurity comprises all the procedures and precautions (both preventive and otherwise) that are taken to protect a herd against infectious diseases – in this case, BVDV. To that end, it is important to get a good picture of the risk factors related to the introduction and spread of BVD infections on a farm.
Working method for farm-specific risk factors
The BVD Prevention Checklist can be used to assess how much of a risk there is for a farm of BVD getting in and spreading. Completing the checklist gives you a BVD risk profile for the farm, with recommendations for further reducing the chance of it being introduced and spreading.
Introduction
The risk factors for BVD getting into a farm show where the risks are for a BVD infection being able to enter. The risk factors for such ingress can then be subdivided into direct and indirect contacts. The risk factors relating to direct contacts:
- Introduction of animals from farms with a lower BVD status.
- Purchasing animals that have been impregnated by a PI animal (‘Trojan’ cattle).
- Shared young stock rearing and calving units.
- ‘Over the wire’ contacts with neighbouring farms that are not BVD-free.
- Visits to cattle and animal shows that are not BVD-free.
Although the lipid envelope of the BVD virus is easily broken down by heat, soaps and organic solvents, indirect contacts can play a role in the epidemiology of BVD. The risk factors relating to indirect contacts:
- People entering the premises (without farm-specific clothing).
- Shared use of livestock transport and materials with other farms (that are not BVD-free).
- Introduction of BVDV via medicine bottles, needles, syringes, gloves, clothing, boots, etc.
- The use of contaminated semen and contaminated embryos.
- Neighbouring non-certified farms.
Contact with wild animals such as deer is estimated to be a very low to insignificant risk factor. Sheep can occasionally be a risk factor (e.g. in the case of shared barns or stalls).
Within-herd transmission
The factors relating to transfers are important within farms both with a BVD-free status and without. It can after all not be excluded that the BVD virus gets reintroduced to a farm with the BVD-free status.
The risk factors relating to internal transfers can again be subdivided into direct and indirect contacts. The risk factors relating to direct contacts:
- Calving management
- Separate calving stalls
- Contacts between calves
- Contact between calves and pregnant cattle
- Animal housing (age groups. sick animals kept apart)
- All-in, all-out
The risk factors relating to indirect contacts:
- Routes walked (from younger to older animals)
- Hand hygiene and clothing for contacts between different age groups
- Animal housing (cleaning and disinfection of stalls after animals are moved):
- When a PI animal is born, large quantities of BVDV are secreted along with the amniotic fluid and this can result in the calving stall becoming infected.
- Stalls and units in which PI animals are housed can be a source of BVDV for a certain interval.
Detecting and disposing of PI animals
A second important measure in the plan for tackling BVD is detecting and as necessary disposing of PI animals on the farm. PI animals secrete large quantities of BVDV continuously throughout their lives. Transiently infected animals secrete much less of the virus and also only do so over for a limited period. PI animals therefore play a crucial role in the epidemiology of BVD. If BVD is to be controlled successfully, these animals need to be detected and disposed of.
Monitoring
Monitoring is the third cornerstone of a successful approach to tackling and controlling BVD. Monitoring is important for determining whether the BVD virus has been eliminated effectively on contaminated farms. It is also important for detecting the introduction of BVDV to farms that were BVD-free.
It is therefore important to monitor the BVD situation on farms that have the BVD-free status or where it is not suspected that BVDV is circulating. Depending on the path chosen, monitoring is done either by virus testing (the ear notch route) or by antibody testing (testing randomly selected young animals or bulk milk).
Vaccination
In addition to the cornerstones of biosecurity, detection and disposal of PI animals and monitoring mentioned above, vaccination can be used as a fourth element for effectively tackling and controlling BVD. Given that BVD has not been eradicated in the Netherlands and PI animals are still being born (at least 457 in 2023), it is not possible to guarantee that the BVD virus will not be introduced or reintroduced on farms with the BVD-free status. In particular at farms with a realistic or high risk of infections, farm-wide vaccination can limit the consequences of introduction of the virus.
Various BVD vaccines are on the market in the Netherlands, based both on inactivated virus and live virus. No DIVA vaccine is available in the Netherlands (as yet). One aim of vaccination is to prevent transplacental infections.
Complete protection by BVDV vaccination is unrealistic, e.g. due to antigenic variation of the BVD virus and the fact that inadequate immune responses can occur in individual animals. In addition, unvaccinated animals may also be present, e.g. after new arrivals or because some animals were missed during the vaccination process.
Effective BVD control therefore always requires biosecurity measures (to prevent introduction of the virus) and monitoring, underpinned by the detection and disposal of PI animals.